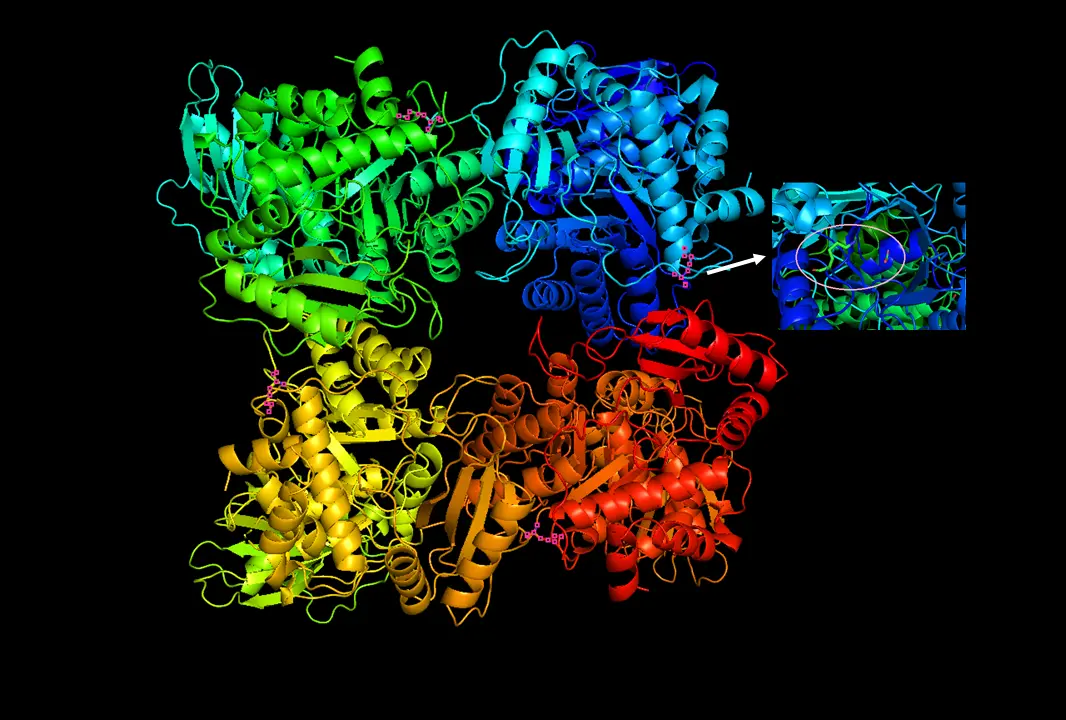


A very old enzyme. Still fixing inorganic carbon in the biosphere through yet another mass extinction. Still grabbing the wrong molecule on occasion. Anyway, here are some more phosphoglycerates.
Kill your lawn, grow a garden. As you do this, look within and do the same.



There was a bit of a drought, but it rained again.


‘Come on, babe. Lots of people are doing it. It’s ok. Totally not psychological torture or psyops. Just one more location. It’ll totally solve the problem. Come on, everyone’s doing it.’


Thank you!! That was very beautiful and quite informative.
Kinda reminds me of Ghana coffin dance and



Lutnick saw The Wire, aspired to be Stringer Bell, and still got it wrong.
https://www.youtube.com/watch?v=U3TMVjgfBW0

You mean Last Week Tonight? The Late Show is Colbert.


Ahhh, thank you.


Unfortunately, Sci-Hub doesn’t have the requested document:
10.1038/s41562-024-02067-4
Rats! Anybody got a pdf?


The on-the-fly meme-making by the Trekkies is positively inspiring.
Lies, Inc. is another by PKD that will leave your head spinning.


Looks like Sepia (Peertube), 藍 ‘Ai’ (Misskey), Mastodon, Fox Tan (Pleroma), and Lemmy.


Was it this one?
https://t.me/liveukraine_media/12694
edit:
More tungsten holes
https://t.me/combatfootageua/13428
Fired from a Leopard
https://t.me/combatfootageua/12291
GMLRS Alternative Warhead Engineer & Manufacturing Development Phase Test & Evaluation
https://www.youtube.com/watch?v=b5h7BkCj5rI
Did you by any chance use trellis netting?
Practice.
Taking notes during lecture helped. Not only does it help cement the information in your mind, it is practice writing legibly enough it can be studied later. You could practice this now, before school starts, by watching something like Khan Academy.
If your major sends you to the whiteboard often, that will help a lot, too. You will naturally improve as you do it out of necessity. Practice on the board until you can write a straight line of consistent text that doesn’t droop or curve down as it goes along.
I second the suggestion for calligraphy in a script you like.
Perhaps practice by trying to quickly write down song lyrics as you listen? I think that’s when I first started to improve.
Pay attention to your classmates who can take good notes quickly. I made a friend who found my writing to be glacially slow, so I watched how they wrote to learn some tricks.
Sorry if some of these won’t help until you’re in, but don’t worry about it too much. I’m sure your handwriting will be markedly improved by the end of even the first year.
p.s.
Write letters or postcards to friends.
Try to fit your favorite quotes on a notecard.
Love it. One example that springs to mind is calling out the NCAA.
“‘Student Ath-uh-letes’? Haha, that is brilliant, sir.”